Noticias

- Título: Zenith® Fenestrated AAA Endovascular Graft FDA approved.
- Fecha: 27-04-2012
This is a brief overview of information related toFDA's approval to market this product. See the links below to the Summary ofSafety and Effectiveness Data (SSED) and product labeling for more completeinformation on this product, its indications for use, and the basis for FDA'sapproval.
Product Name: Zenith® Fenestrated AAA Endovascular Graft (with the adjunctiveZenith Alignment Stent)
Manufacturer: Cook Incorporated
Address: 750 Daniels Way, PO Box 489, Bloomington, IN 47402
Approval Date: April 4, 2012
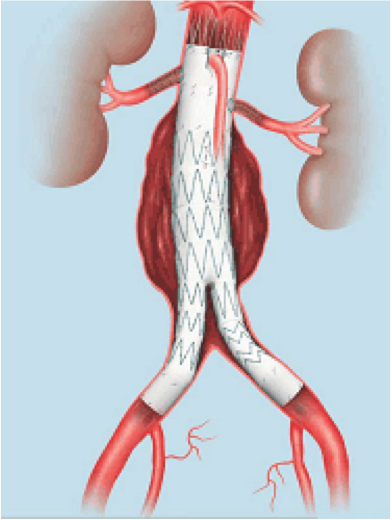
Approval Letter: http://www.accessdata.fda.gov/cdrh_docs/pdf2/p020018s040a.pdfWhat is it? The Zenith® Fenestrated AAA Endovascular Graft (with the adjunctiveZenith Alignment Stent) is an endovascular stent graft used to repair abdominal aortic aneurysms or aneurysms that involve both the abdominal aortaand iliac arteries (the large arteries that supply blood to the pelvis andlegs).
An abdominal aortic aneurysm (AAA) is a bulge that occurs in the body's largestartery (the aorta) as it passes through the abdomen. The bulge is caused by aweakening or thinning in the wall of the artery. A synthetic tube-like device (agraft) is used within the blood vessel (endovascular) to treat the AAA bysealing it off.
The Zenith® Fenestrated AAA Endovascular Graft ismade of a fabric tube supported by a metal framework. The fabric has carefullypositioned holes to allow blood to continue to flow to the body's organs. Thegraft has three parts: an upper “main body”, a lower “main body” and one “leg.”It is made of a polyester material attached to a frame of stainless steelstents (or scaffolds). The stents support the graft and hold it open within theblood vessel. The adjunctive Zenith Alignment Stent is made of stainless steeland is used to help keep the holes in the graft lined up with the arteries thatgo to the organs.
How does it work?
The graft is delivered to the aneurysm in the aortaby way of a long, flexible delivery tube. The delivery tube containing thegraft is inserted through a small incision in the groin where it is threadedthrough a blood vessel and advanced to the location of the aneurysm. The doctoruses a type of x-ray (fluoroscopy) to guide the graft. The graft is attached to thewall of the aorta by the self-expanding stents. Blood flow can then continuethrough the aorta without filling the aneurysm. This is intended to preventfurther growth and possible rupture of the aneurysm.
Stents may be placed through the holes in the graftto help keep the blood flowing into the arteries that supply the organs.
When is it used? The Zenith® Fenestrated AAA Endovascular Graft (with the adjunctiveZenith Alignment Stent) is used instead of an open (more invasive) surgery inpatients who have a n abdominal aortic aneurysm or aneurysms that involve theaorta and the iliac arteries.
What will it accomplish? The Zenith® Fenestrated AAA Endovascular Graft(with the adjunctive Zenith Alignment Stent) should benefit patients with anabdominal aortic aneurysm or aneurysms that involve the aorta and the iliacarteries by preventing further growth and rupture of the aneurysm.
When should it not be used? The Zenith® Fenestrated AAA Endovascular Graft(with the adjunctive Zenith Alignment Stent) should NOT be used inpatients:
- who have a condition that threatens to infect the graft
- who have sensitivities or allergies to the device materials or,
- who are unable to undergo the necessary preoperative and postoperative imaging and post-implantation studies.
Additional information: The Summary of Safety andEffectiveness Data and labeling are available online.





