Noticias

- Título: Success of the first 4 Spanish VasQ clinical cases, an external support device for AV fistulas
- Fecha: 06-09-2017
Tel Aviv, Israel, Aug. 04. 2017 – Laminate Medical Technologies Ltd., a medical device company focused on VasQ, an external support device for AV fistulas, to be implanted during the fistula creation surgical procedure, today announced, the success of the first 4 Spanish clinical cases.
The cases have been performed by Dr. Gaspar Mestres at Hospital Clinic of Barcelona, Vascular Surgery Division, which is led by Dr. Vicente Riambau
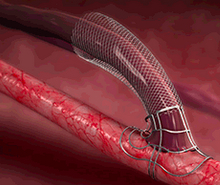
The VasQ targets the two main fistula failure modes:
Turbulent flow around the area of connection and increased venous wall tension due to the exposure to arterial circulation conditions. Disturbed fistula hemodynamics result in pathological thickening of the inner layer of the vein that leads to thrombosis and stenosis, a narrowing of the blood vessel.
Dr. Vicente Riambau, head of the department stated:
“We are very glad to participate in the VALUE international study and collect robust clinical data of the VasQ outcomes! We think this is a promising technology”.
Dr. Mestres, who performed the surgeries said:“VasQ is very easy to implant, with minimal change of the regular fistula procedure and in no way increasing time or difficulty of the AVF creation surgery. Also the use of VasQ has a really easy learning curve”. He added that the conceptof VasQ is very interesting and that giving the AVF anastomosis the proper angulation and fixation as well as optimizing the flow patterns can significantly improve AVF.
“After receiving IDE approval from FDA to initiate clinical study of the VasQ device in the US and reimbursement approval in Germany, this is the next important and strategic milestone. This will allow more ESRD patients and Drs. to benefit from the tremendous promising results which VasQ showed in our multinational randomized controlled trial” said CEO Tammy Gilon and added that improved functional maturation of fistulas with VasQ are a key aspect of this results.
VasQ is available in Spain through Laminate Medical´s distribution partner Salutaria Universal S.L. in Barcelona.
Laminate Medical Technologies Ltd. is a Tel Aviv,Israel based medical device company focused on the $ 1B market for AVF procedures.The company has European CE approval for VasQ which has been specifically designed to be implanted during the AVF procedure and improving maturation success and AVF patency rates.
For more information, please visitwww.laminatemedical.com.
- Fuente: endovascular.es





