Noticias

- Título: First Commercial Placements of the Angel® Catheter in the United States
- Fecha: 05-10-2016
GOLDEN, Colo., Oct. 5, 2016 /PRNewswire/ -- Bio2 Medical is pleased to announce that the Angel® Catheter, a new device for the prevention of pulmonary embolism (PE), was placed in two patients at St. Mary's Medical Center in West Palm Beach, Florida, by Lawrence Lottenberg, MD FACS and Robert Borrego, MD FACS on Thursday, September 29, 2016. This is the first commercial usage of the Angel® Catheter, and St. Mary's Medical Center of Tenet Health is the first hospital in the United States to place the device. The Angel® Catheter received 510(k) clearance from the United States Food and Drug Administration (FDA) as the first ever, prophylactic indication for a medical device to protect critically ill patients at high-risk for pulmonary embolism and contraindicated for anticoagulation.
The first patient was at high risk for PE due to traumatic brain injury post craniotomy. The second patient had prolonged immobilization due to a cervical spinal cord injury and quadriplegia. Both patients were contraindicated to prophylactic anticoagulation for 7-10 days. The Angel® Catheter was placed at the bedside in the trauma ICU, without the need for fluoroscopic guidance, allowing Dr. Lottenberg and Dr. Borrego to provide their patients with immediate PE prophylaxis.
Dr. Lottenberg stated: 'The Angel® Catheter provides an unmet need for a prophylactic indication for the prevention of pulmonary emboli. Unlike traditional filters, the Angel® Catheter cannot migrate from its deployment position, and it is easily inserted and easily removable for patients who cannot be prophylactically anticoagulated during the immediate and early phases of injury. There are none of the complications seen in the many currently available inferior vena cava filters. This makes the device unique and one of a kind. The recognition of the value of this technology to patient safety and patient care by the Tenet Healthcare System and St. Mary's Hospital in bringing this technology to our Level I trauma cannot be overstated.'
Dr. Borrego provided the following statement: 'The placement of the Angel® Catheter at the bedside is a safe and efficient way to protect patients at high risk for pulmonary embolism, who cannot be anticoagulated for seven to ten days therefore protecting them from additional morbidity / mortality due to pulmonary embolism in high risk patients.'
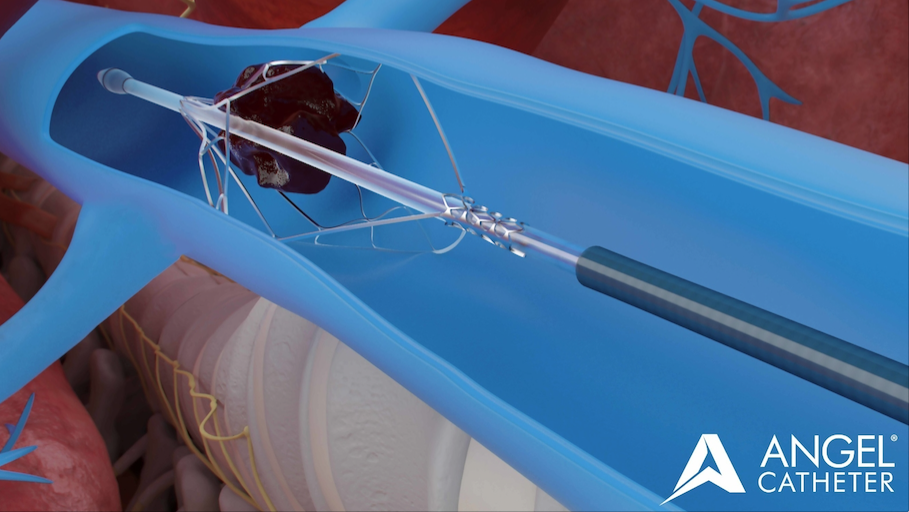
Intended Use of the Angel® Catheter
- The Angel® Catheter is intended to provide the combined functions of an inferior vena cava (IVC) filter and a multi-lumen central venous catheter.
- The Angel® Catheter is intended for short-term use for the prevention of clinically significant pulmonary embolism (PE) in critically ill patients at high risk for PE or recurrent PE, and recognized contraindications to standard pharmacological thromboprophylaxis therapy.
- The Angel® Catheter is also intended to provide access to the central venous system.
Details of First Commercial Placement
Hospital:
St. Mary's Medical Center, West Palm Beach, FL
Hospital system:
Medical School Affiliation:
Florida Atlantic University
Boca Raton, FL
Physicians:
Director of Trauma Research and Education
Trauma and Acute Care Surgeon
St. Mary's Medical Center
West Palm Beach, FL
Affiliate Clinical Associate Professor of Surgery
Director of Surgical Simulation and Technology
Charles E. Schmidt College of Medicine
Florida Atlantic University
Boca Raton, Florida
Director of Trauma & Surgical Critical Care
St. Mary's Medical Center
West Palm Beach, Florida
Affiliate Clinical Associate Professor of Surgery
Charles E. Schmidt College of Medicine
Florida Atlantic University
Boca Raton, Florida
Affiliate Clinical Associate Professor of Surgery
University of Vermont Medical School
Burlington, Vermont
Learn more about Bio2 Medical and the Angel® Catheter by visiting www.bio2medical.com. To request a packet containing all clinical evidence to-date, please contact sbrewer@bio2medical.com.
Photo - http://photos.prnewswire.com/prnh/20161005/415475
SOURCE Bio2 Medical, Inc.
Related Links
- Fuente: endovascular.es





