Noticias

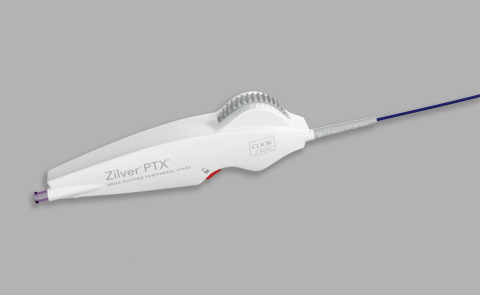
- Título: Zilver® PTX® with Thumbwheel Delivery System Launched in France
- Fecha: 22-09-2016
LIMERICK, Ireland--(BUSINESS WIRE)--Cook Medical launches Zilver® PTX® drug-eluting peripheral stent thumbwheel delivery system in France. The French Health Ministry has now approved reimbursement for the Zilver PTX thumbwheel delivery system.
The rotating thumbwheel system, which is available for purchase in approved markets across Europe, provides deployment for the world’s first drug-eluting stent for the superficial femoral artery (SFA).
The thumbwheel design features include an ergonomic handle that facilitates single-handed deployment and is available with stents up to 120 mm in length.
About Zilver PTX:
- Zilver PTX received its initial CE Mark in 2009 and FDA approval in 2012.
- 5-year data proves that Zilver PTX when compared to Zilver bare-metal stents reduces restenosis and reinterventions.1
- With its proven drug effect, Zilver PTX inhibits neointimal hyperplasia. 1,2
- Paclitaxel helps maintain patency by working to prevent restenosis. 2
1 Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation. 2016;133(15):1472-83.
2 Dake MD, Van Alstine WG, Zhou Q, et al. Polymer-free paclitaxel-coated Zilver PTX Stents—evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011;22(5):603-610.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials, and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees, and our communities. Find out more at www.cookmedical.eu, and for the latest news, follow us on Twitter and LinkedIn.
Contacts
Cook Medical
Kacey Martin, +812-339-2235, ext. 105164; +812-320-3828 (mobile)
Content Specialist, PR and Social Media
kacey.martin@cookmedical.com- Fuente: endovascular.es





