Noticias

- Título: Positive 12-month TOBA-BTK results presented at SCAI 2016
- Fecha: 04-05-2016
Data Presented at the Society for Cardiac Angiography and Interventions 2016 Annual Meeting Demonstrate Sustained Vessel Patency and Low Re-Intervention Rates with the Tack Endovascular System™
May 04, 2016 05:00 PM Eastern Daylight Time
WAYNE, Pa.--(BUSINESS WIRE)--Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced that positive twelve-month results from its Tack Optimized Balloon Angioplasty – Below-the-Knee (TOBA-BTK) clinical study were presented at the SCAI 2016 conference by Dr. Christian Wissgott, Assistant Director, at Westküstenklinikum Heide in Heide, Germany.
“The Tack Endovascular System holds much promise for improving the long term outcomes associated with balloon angioplasty below the knee. I look forward to building on these exciting results in the expanded TOBA II BTK clinical trial that is currently being planned.”
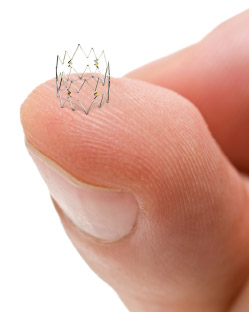
The TOBA-BTK study enrolled 35 subjects at 6 sites in Europe and New Zealand. All study subjects were suffering from critical limb ischemia, a form of peripheral arterial disease, in one or both legs. Of the subjects enrolled, 32 were treated with the 4 French Tack Endovascular System following standard balloon angioplasty in the tibial and peroneal arteries. The Tack Endovascular System is a new technology designed to repair dissections (or tears) in the artery wall that frequently occur as a complication of balloon angioplasty. The system allows physicians to repair these dissections while leaving a minimal amount of foreign material in the artery, reducing mechanical stress on the artery, and preserving future treatment options.
Some of the key conclusions from the TOBA-BTK study included:
- 78.4% 12-month primary patency
- 89.2% 12-month primary assisted patency
- 93.5% freedom from target lesion revascularization (TLR)
- 84.5% amputation-free survival
- 87.1% freedom from major amputation
Dr. Wissgott stated, “Restoring blood flow to the legs in patients with critical limb ischemia is vitally important to their quality of life and survival. We have few treatments that have a demonstrated ability to keep the small arteries below the knee open over 12 months, so these TOBA-BTK results are truly encouraging. These data suggest that repairing arterial dissections following angioplasty below the knee, using the minimal metal design approach of the TackTM implant, can keep the treated arteries open and blood flowing.”
Patients in the study demonstrated a marked improvement, as measured by the Rutherford Classification System, a 7-stage scale ranging from no symptoms (class 0) to gangrene (class 6). The study data suggest that an increasing number of patients continue to improve, with 75% of patients experiencing a 4 or 5 step improvement in Rutherford Classification from baseline (p<0.0001) at 12 months.
Dr. Wissgott continued, “The Tack Endovascular System holds much promise for improving the long term outcomes associated with balloon angioplasty below the knee. I look forward to building on these exciting results in the expanded TOBA II BTK clinical trial that is currently being planned.”
Peter Schneider, M.D., Chief of Vascular Therapy at the Kaiser Foundation Hospital in Honolulu, and Intact Vascular’s Founder and Chief Medical Officer, added, “The existing treatments for arterial dissections below the knee involve leaving a great deal of metal behind, and in the small arteries of the lower leg that is plagued with problems. The Tack implant takes a very novel approach and allows the treating physician to target therapy only where it is needed to promote healing of the injured artery.”
Based on these promising results, the company is pursuing an expanded study (TOBA II BTK) that will assess the performance of the Tack Endovascular System in a larger population and will include U.S. and international investigators.
About Intact Vascular
Intact Vascular is a privately held medical device company that develops minimally invasive peripheral vascular products. The Tack Endovascular System is designed to optimize peripheral balloon angioplasty results in the treatment of peripheral arterial disease. Visit www.intactvascular.com for more information.
This press release contains “forward-looking statements” concerning the development of Intact Vascular's products, the potential benefits and attributes of such products, and the company’s expectations regarding its prospects. Forward-looking statements are subject to risks, assumptions and uncertainties that could cause actual future events or results to differ materially from such statements. These statements are made as of the date of this press release. Actual results may vary. Intact Vascular undertakes no obligation to update any forward-looking statements for any reason.
Tack Endovascular System™ and Tack™ are trademarks of Intact Vascular, Inc.
The 6 French Tack Endovascular System™ is CE Mark Authorized under EC Directive 93/42/EEC. (4 French pending)
Not Available for sale or use in the United StatesContacts
Intact Vascular, Inc.
Andrea Dunkle, 1-484-253-1048- Fuente: endovascular.es





