Noticias

- Título: Lombard Medical Provides Update on New Altura® Endovascular Stent Graft Launch
- Fecha: 04-04-2016
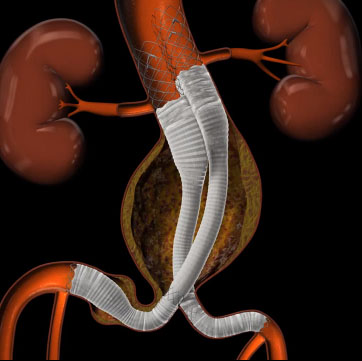
IRVINE, Calif., April 04, 2016 (GLOBE NEWSWIRE) -- Lombard Medical, Inc. (NASDAQ:EVAR), a medical device company focused on Endovascular Repair (EVAR) of abdominal aortic aneurysms (AAAs), today announced an update on the commercial launch of the new Altura® Endovascular Stent Graft. Since the commercial launch in late February, Altura has been implanted at approximately half of the 20 initial launch reference centers identified in the United Kingdom (UK) and Germany. The remaining reference centers have Altura cases planned for early in the second quarter. Early experience supports the Company’s belief that Altura offers a simplified, accurate, fast and predictable solution for the treatment of AAAs in standard anatomy.
One of the most experienced launch reference centers to date is the University Hospital of South Manchester (UHSM) in the UK, where physicians have already demonstrated the use of Altura as a “day case” (outpatient) treatment for AAA disease where the patient is admitted to the hospital, treated and discharged home on the same day.
Steven Richardson, MD, Consultant Vascular and Endovascular Surgeon at UHSM, commented, 'Since introducing the new Altura stent graft into our clinical practice, we have already been able to perform day case endovascular aneurysm repair. The unique features of Altura provide a predictable procedure time and allow for a minimally invasive percutaneous approach which greatly benefits the patient and the hospital system.”
“Establishing our UK and Germany reference centers is an important step in executing our Altura commercial launch strategy,” said Lombard CEO Simon Hubbert. “We continue to experience a growing number of CT scans for evaluation, and we are looking to build on the growing interest in Altura as a new mainstream AAA endovascular device with the potential to deliver faster procedure times and shorter hospital stay for many patients.”
About Lombard Medical, Inc.
Lombard Medical, Inc. is an Irvine, CA-based medical device company focused on the $1.7bn market for minimally invasive treatment of abdominal aortic aneurysms (AAAs). The Company has global regulatory approval for Aorfix, an endovascular stent graft which has been specifically designed to treat patients with the broadest range of AAA anatomies, including aortic neck angulation up to 90 degrees. The Company has also achieved CE Mark for the Altura™ endograft system, an innovative ultra-low profile endovascular stent graft that offers a simple and predictable solution for the treatment of more standard AAA anatomies. Altura was launched in Europe in January 2016, with an international rollout planned for later in 2016. For more information, please visit www.lombardmedical.com.Forward-Looking Statements
This announcement contains forward-looking statements that reflect the Company’s current expectations regarding future events. These forward-looking statements generally can be identified by the use of words or phrases such as “believe,” “expect,” “future,” “anticipate,” “look forward to,” “intend,” “plan,” “foresee,” “may,” “should,” “will,” “estimates,” “outlook,” “potential,” “optimistic,” “confidence,” “continue,” “evolve,” “expand,” “growth” or words and phrases of similar meaning. Statements that describe objectives, plans or goals also are forward-looking statements. Forward-looking statements are subject to risks, management assumptions and uncertainties. Actual results could differ materially from those projected herein and depend on a number of factors, including the success of the Company’s research and development and commercialization strategies, the uncertainties related to the regulatory process and the acceptance of the Company’s products by hospitals and other medical professionals, the uncertainty of estimated revenues and profits, the uncertainty of current domestic and international economic conditions that could adversely affect the level of demand for the Company’s products and increased volatility in foreign exchange rates, the inability to raise additional funds, and the risks, uncertainties and other factors described under the heading “Risk Factors” in the Company’s Form 20-F filed with the Securities and Exchange Commission dated April 29, 2015. Readers are urged to consider these factors carefully in evaluating the forward-looking statements. The forward-looking statements included herein are made only as of the date of this report and the Company undertakes no obligation to update these statements in the future.- Fuente: endovascular.es





