Noticias

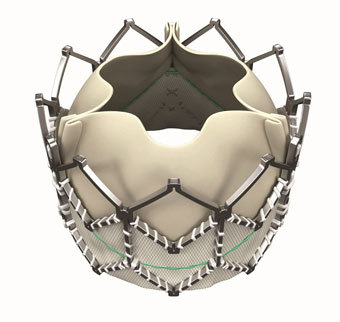
- Título: FDA Approves Edwards' Sapien XT for Pulmonary Valve Replacement
- Fecha: 02-03-2016
The agency has considerably expanded the indications of Edwards Lifesciences’ Sapient XT transcatheter valve, which was granted FDA approval for use in valve-in-valve transcatheter aortic valve replacement (TAVR) procedure.
Nancy Crotti
FDA’s approval of the XT TAVI valve will allow for minimally invasive surgery on adults and children who have a narrowed pulmonary valve, or moderate or greater pulmonary regurgitation caused by congenital heart disease, according to astatement from the Irvine, CA, company.
Until now, these patients have had to endure multiple open-heart surgeries, often beginning at birth, the company said.
“As risks increase with each open-heart surgery, a non-surgical option can help them receive treatment, recover and return to normal activities sooner," added Larry Wood, Edwards' corporate vice president, transcatheter heart valves.
Data from the COMPASSION (COngenital Multicenter trial of Pulmonic vAlve regurgitation Studying the SAPIEN InterventIONal THV) clinical trial and from European clinics figured into FDA’s decision.
Physicians working with Edwards technology performed the first U.S. transcatheter pulmonic case on a compassionate basis in December 2005. Two years later, FDA conditionally approved an investigational device exemption clinical trial.
This FDA approval marks just the latest success for Edwards’ TAVR, a technology it helped pioneer. The company was the first in the United States to launch TAVR technology and has been able to maintain market share with the launch of Edwards’ Sapien 3 valve in the United States. Transcatheter heart valves sales in 2015 were up 38%, to $1.2 billion.
Edwards estimates that the approval will generate between $50 million and $100 million, CEO Mike Mussallem told investors in a recent conference call. While it’s a small opportunity for the company, “it’s really important for these patients in need,” he said. “These are often children.”
Edwards plans to begin enrolling patients in a clinical trial for the SAPIEN 3 valve in pulmonic in the second quarter of 2016.
- Fuente: endovascular.es





