Noticias

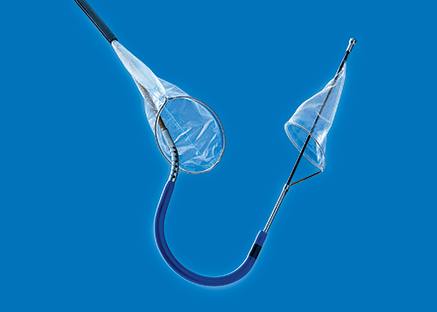
- Título: Claret treats first patient in pivotal US trial of Sentinel cerebral protection system
- Fecha: 08-10-2014
US-based Claret Medical has completed treatment of the first patient in its multicentre pivotal SENTINEL trial of the Sentinel cerebral protection system (CPS).
A total of 284 patients at about 15 centres in the US, will be enrolled in the prospective, randomised, controlled, blinded SENTINEL trial, which is designed to evaluate the role of cerebral protection during transcatheter aortic valve replacement (TAVR).
The SENTINEL trial is claimed to be the first in the US to evaluate capture and removal of debris released during TAVR that may otherwise be the source of stroke.
A national co-principal investigator for the trial, Susheel Kodali, has treated the first patient at New York and Presbyterian Hospital/Columbia University Medical Center.
The trial's primary endpoints are the reduction in total new lesion volume as determined by diffusion-weighted magnetic resonance imaging (DW-MRI) and major adverse cardiac and cerebrovascular events (MACCE).
A number of secondary endpoints, including neurocognitive and histopathological outcomes during TAVR, will be compared in the trial arms with and without cerebral protection.
Cleveland Clinic Sones Cardiac Catheterization Laboratories director and national co-principal investigator for the trial Samir Kapadia said: "Any occurrence of stroke is one too many, and results from this clinical trial may give us the evidence needed to make cerebral protection a standard of care during TAVR, as it is in carotid artery stenting.
"By both capturing and removing embolic debris released during TAVR, the Sentinel CPS may offer a unique neuroprotective benefit.
"We expect the device to demonstrate a similarly significant reduction in the number and size of lesions in the brains of TAVR patients when cerebral protection is used as was recently reported in the CLEAN-TAVI trial."
The company recently reported 30-day results from the CLEAN-TAVI randomised, controlled trial evaluating its cerebral protection system.
The results showed 53% reduction in the total volume of new brain lesions and 60% reduction in the number of new brain lesions two days after the TAVR procedure when using the company's cerebral protection system.
- Fuente: endovascular.es





