Noticias

- Título: Covidien Announces European Launch of Pipeline™ Flex Embolization Device
- Fecha: 24-06-2014
Minimally-invasive flow diversion device for unruptured brain aneurysms designed for more accurate, controlled placement1
DUBLIN, Ireland--(BUSINESS WIRE)--Jun. 24, 2014-- Further strengthening its broad line of neurovascular products to treat unruptured brain aneurysms, Covidien plc (NYSE:COV) announced the European launch of its Pipeline™ Flex embolization device at the annual Live Interventional Neuroradiology & Neurosurgery Course (LINNC), held in Paris June 23-25. This next-generation flow diversion device received CE Mark earlier this year.
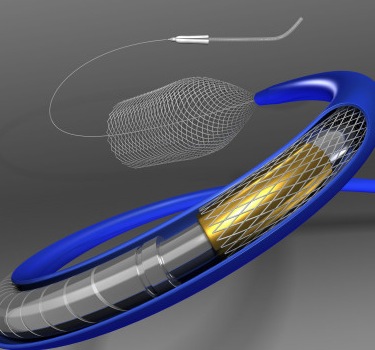
Covidien’s Pipeline™ Flex embolization device is the next advancement in flow diversion in Europe. (Photo: Business Wire)
Designed to divert blood flow away from an aneurysm, the Pipeline Flex device features a braided cylindrical mesh tube that is implanted across the base or neck of the aneurysm. The device cuts off blood flow to the aneurysm, reconstructing the diseased section of the parent vessel. The device is repositionable and designed for even more accuracy and controlled placement1. Among other features, it includes an instant braid release system that makes it even easier to place1.
“The Pipeline Flex embolization device is the next advancement in flow diversion, combining our clinically-proven braid design2 with a new delivery system designed to offer even more accuracy and control when performing these advanced procedures inside the brain,” said Brett Wall, president, Neurovascular, Covidien. “Covidien is dedicated to seeking customer feedback and advancing product design to meet their needs.”
In Europe, the Pipeline Flex device is intended for the endovascular embolization of cerebral aneurysms. According to the Brain Aneurysm Foundation, there are nearly 500,000 deaths worldwide each year caused by brain aneurysms and half the victims are younger than 50 years of age.3
The first-generation Pipeline™ embolization device has been used to treat patients in Europe since 2009. It has been the only flow diversion device commercially available in the U.S. since it was approved by the U.S. Food and Drug Administration in April 2011. The Pipeline Flex device is not currently approved for use in the U.S.
For more information about the Pipeline device platform, please visit:
www.covidien.com/pipelineinfo- Fuente: endovascular.es





