Noticias

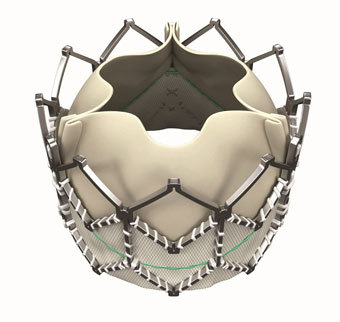
- Título: Edwards Lifesciences Launching SAPIEN XT Valve In The U.S.
- Fecha: 16-06-2014
IRVINE, Calif., June 16, 2014 /PRNewswire/ -- Edwards Lifesciences Corporation (NYSE: EW), the global leader in the science of heart valves and hemodynamic monitoring, today announced that it has received United States Food and Drug Administration (FDA) approval for its Edwards SAPIEN XT transcatheter aortic heart valve for the treatment of high-risk and inoperable patients suffering from severe symptomatic aortic stenosis (AS). This next-generation, lower-profile system, which includes the 29mm valve size for patients with a large native annulus, will allow for the treatment of more patients.
The Edwards SAPIEN XT valve will be immediately available to patients at leading cardiovascular centers across the nation, along with the NovaFlex+ transfemoral delivery system that can be delivered through a low-profile 16-French expandable sheath (eSheath) and the Ascendra+ transapical and transaortic delivery systems.
"There is a substantial and growing body of evidence that the SAPIEN XT valve benefits both high-risk and inoperable patients, and clinicians have documented these consistently positive results in both randomized studies and European country registries," said Martin B. Leon, MD, director, Center for Interventional Vascular Therapy at NewYork-Presbyterian/Columbia University Medical Center and professor of medicine at the Columbia University College of Physicians and Surgeons. Dr. Leon was the co-principal investigator for the PARTNER II Trial, which was Edwards' second randomized controlled trial of a transcatheter valve and evaluated the SAPIEN XT valve. "The results from the PARTNER II Trial in treating U.S. inoperable patients with the SAPIEN XT valve demonstrated a reduction in complications with the TAVR procedure, and improved patient outcomes over earlier trials."
"Clinicians have demonstrated their confidence in the SAPIEN valves by making them the market-leading transcatheter valves, and this approval provides greater options for U.S. patients who can benefit from the substantial enhancements in this proven platform," said Larry L. Wood, Edwards' corporate vice president, transcatheter heart valves. "The SAPIEN family of valves has been used in the treatment of more than 70,000 patients globally – with the majority of those patients treated with SAPIEN XT – and we look forward to Heart Teams across America transforming the lives of even more patients with this advanced transcatheter valve."
The Edwards SAPIEN XT valve has been commercially available in Europe since 2010, and received regulatory and reimbursement approval in Japan in 2013.
- Fuente: endovascular.es





