Noticias

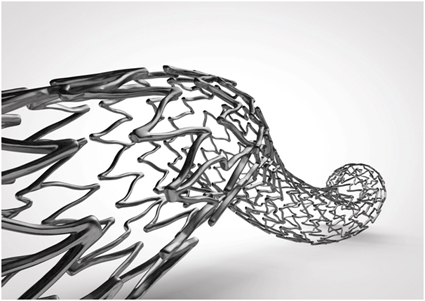
- Título: Veryan to Present Two-Year Results from Mimics Randomised Controlled Study of BioMimics 3D® Stent at New Cardiovascular Horizons Conference
- Fecha: 08-05-2014
- HORSHAM, UK and GALWAY, Ireland, 8th May 2014 – Veryan Medical, a company developing highly innovative biomimetic stents with improved hemodynamics and vascular compatibility, today announced that the two-year results of the Mimics randomized controlled study with the BioMimics 3D Stent System will be presented at the 15th Annual New Cardiovascular Horizons (NCVH) Conference taking place between May 28th and 30th in New Orleans. Principal Investigator Professor Thomas Zeller, Head of the Department of Angiology at the Universitäts Herzzentrum, Freiburg-Bad Krozingen, Germany, will present the data during the New Technology session on Friday, May 30th.The BioMimics 3D nitinol stent incorporates Veryan’s patented 3D helical centreline, an advanced stent design that promotes natural swirling blood flow to elevate wall shear stress in the stented segment. Preclinical studies have shown that stents with 3D helical curvature significantly reduce the formation of neointimal hyperplasia compared to straight stents. The helical curvature of the BioMimics 3D stent is also designed to help the stented vessel mimic the natural biomechanical performance of the femoropopliteal artery during knee flexion, mitigating the risk of stent fracture and vessel kinking.The Mimics study is a prospective, randomised, multicentre controlled trial conducted at eight German investigational centers. A total of 76 patients were enrolled and randomized 2:1 (50 BioMimics 3D v 26 Control) in patients undergoing femoropopliteal artery intervention. Supported by an independent core lab, Mimics’ investigators compared the safety, efficacy and vascular hemodynamics of the BioMimics 3D stent to those of a straight nitinol stent (24/26 control subjects were treated with C.R. Bard’s LifeStent).Previously presented Mimics’ data have provided evidence for a patency-protective effect in patients treated with BioMimics 3D. These preliminary data showed a clear separation favouring BioMimics 3D over control stents in the two-year Kaplan Meier survival estimates for freedom from clinically driven target lesion revascularization (TLR). Prof. Zeller’s presentation at the New Cardiovascular Horizon Annual Conference will provide the first disclosure of the full two-year data set from this important comparative study.“Mimics’ data have continued to suggest that the flow effects produced by the helical design are contributing to an improved outcome compared to that achieved with the straight control stent. I will present the final two-year primary patency and TLR results at the NCVH meeting, and use evidence from the Mimics database to differentiate the performance characteristics of a biomimetic stent from those of a straight stent”, commented Professor Zeller.“We are very grateful to Professor Zeller and the Mimics Investigators for the support that has enabled Veryan to complete this benchmark evaluation of our biomimetic 3D helical stent technology. We believe this advanced stent design offers outstanding benefits in femoropopliteal use and also has potential for innovation in many other areas of endovascular intervention. Presentation of the two-year Mimics’ results represents a major step forward in this endeavour”, added Veryan Chief Executive Chas Taylor.Veryan has received CE Mark approval for the BioMimics 3D stent and plans to commercialise the stent later this year.
- Fuente: endovascular.es





