Noticias

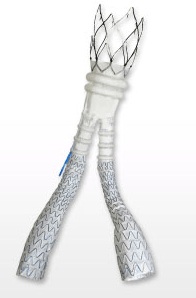
- Título: TriVascular Completes Enrollment of the OVATION® European Post-Market Registry
- Fecha: 27-01-2014
500-patient, real-world study of innovative, lowest profile EVAR system bolsters strong library of clinical evidence
Santa Rosa, CA, January 27, 2013 – TriVascular, Inc. today announced the enrollment completion of the OVATION post-market registry, a 500-patient, 30-center European study. The multicenter prospective post-market registry evaluates the safety and performance of the Ovation and Ovation PrimeTM Abdominal Stent Graft Systems, with patient follow-up out to five years. The study is evaluating the treatment of abdominal aortic aneurysms (AAA) in the real-world setting of routine clinical practice. Including the Ovation pre-market clinical trial, over 700 patients have participated in a clinical study with either the Ovation or Ovation Prime stent graft system. The 30-day OVATION post-market study results will be presented by Professor Dr. Dierk Schienert at the Leipzig Interventional Course (LINC) 2014 meeting in Germany on January 28, 2014.
“I am pleased to be partnering with TriVascular on this important study to generate clinical evidence of that scope,“ said Professor Dr. Dierk Scheinert, Head of the Department of Medicine, Angiology and Cardiology at Park-Krankenhaus Leipzig, Germany, who serves as the OVATION European Principal Investigator. “We have been very pleased with our Ovation experience, both in challenging and more straightforward anatomies. Since our participation in the original CE Mark study, we have seen excellent results with the system and it is now a routine part of our treatment protocol for AAA. I look forward to the results of this study, which will allow us to evaluate the Ovation system in routine clinical use.”
The post-market study’s primary endpoint is treatment success, a composite of technical and clinical success at 12 months. Technical success includes successful delivery and deployment of the stent graft. Clinical success includes freedom from aneurysm expansion, aneurysm rupture, Type I and III endoleaks, conversion to open repair, stent graft migration, and stent graft occlusion. In the pre-market pivotal trial, at both the 1-year and 2-year mark, the Ovation system demonstrated 100% freedom from Type I and III endoleaks, migration, rupture and conversion to open surgical repair. In addition, the pivotal trial cohort showed no aortic neck growth at either 1 or 2 years. Forty eight percent (48%) of the patients in the trial were treated via a percutaneous (PEVAR) vessel access method. Thirty nine percent (39%) of the patients treated in the Ovation pivotal study would have been excluded from treatment in previous EVAR trials due to challenging anatomical characteristics. One-year results from the Ovation pre-market trial were published in the Journal of Vascular Surgery in January 2014.
“We are excited to have completed enrollment in the OVATION European study,” said Christopher G. Chavez, Chairman and CEO of TriVascular. “Generating clinical evidence to support our platform is a core strategy for TriVascular. We believe the Ovation system allows more patients to gain access to an improved EVAR solution, and that studies like the European registry allow us to prove that point. I am grateful to our clinical partners for their
At 14F OD, the Ovation stent graft is the lowest profile, commercially available EVAR system. With the broadest indications for use of any platform, it is designed to safely expand the pool of patients eligible for EVAR by addressing a wider range of diseased anatomies. Today, the Ovation system is approved for sale in over 30 countries and, worldwide, over 3,000 patients have been treated with the Ovation or Ovation Prime stent graft systems.
- Fuente: endovascular.es





