Noticias

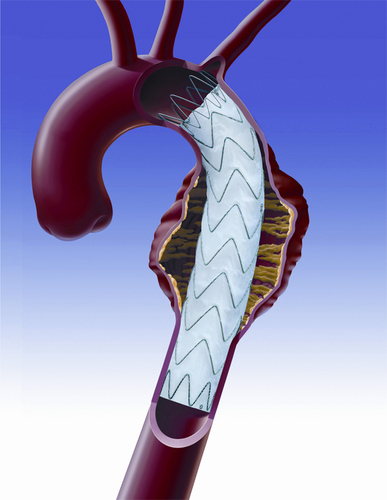
- Título: Valiant Captivia Thoracic Stent Graft System From Medtronic Receives FDA Approval for Treating Aortic Dissections
- Fecha: 28-01-2014
MINNEAPOLIS -- Jan. 28, 2014 -- Continuing to expand the role of endovascular aortic repair, Medtronic, Inc. (NYSE: MDT) has received approval from the U.S. Food and Drug Administration (FDA) for the Valiant Captivia Thoracic Stent Graft System to be used in the treatment of type B aortic dissections, a serious cardiovascular condition associated with high morbidity and mortality in which the upper segment of the body's main artery has become torn along the innermost layer of the vessel wall.
Supported by the results of the U.S. Medtronic DISSECTION trial, the new indication expands treatment options for this challenging patient population by providing physicians with a minimally invasive alternative to open surgical repair and medical therapy.
'Acute type B aortic dissection is a potentially life-threatening condition that historically has been treated with either medical therapy or, when necessary, through invasive surgical techniques,' explained Joseph Bavaria, MD, professor of surgery and director of the thoracic aortic surgery program at the University of Pennsylvania in Philadelphia, and a national principal investigator for DISSECTION.
'The trial we conducted shows that endovascular repair with the Valiant Captivia System provides a safe, effective and potentially life-saving treatment option for acute dissection patients.'
Trial Results
Presented by Dr. Bavaria yesterday at the 2014 annual meeting of the Society for Thoracic Surgery, 12-month data from the 50 patients evaluated in Dissection demonstrate safety and efficacy of the Valiant Captivia System in the treatment of dissections, with excellent technical success.Conducted at 16 U.S. sites, the trial met its primary safety endpoint by achieving an 8 percent all-cause mortality rate at 30 days, which represents a three- to four-fold mortality improvement over open surgical repair.[1],[2] Additionally, 100 percent technical success and 100 percent coverage of the primary entry tear at implant were achieved in the trial.
Rodney White, MD, chief of vascular surgery at Harbor-UCLA Medical Center in Torrance, Calif., and the trial's leading enroller, added: 'Data out to one year continue to show positive aortic remodeling of the stented segment, with a 100 percent increase in true lumen volume and no ruptures.'
Indicated for a variety of thoracic aortic lesions, the Valiant Captivia System features a unique proximal tip-capture mechanism, which enables controlled deployment and accurate placement of the stent graft. Based on independent conformability bench testing of multiple thoracic stent grafts, the Valiant stent graft is the only device that maintains complete apposition to the vessel wall regardless of angulation or oversizing. Since its initial 2005 launch in Europe, the Valiant stent graft has been implanted in about 50,000 patients worldwide -- more than any device of its kind.
- Fuente: endovascular.es





