Noticias

- Título: Gore Launches Early Cannulation Capable GORE® ACUSEAL Vascular Graft
- Fecha: 19-11-2013
19 November2013 (Flagstaff, Arizona and New York)
Results ofearly cannulation clinical trial presented at the 2013 VEITHsymposium
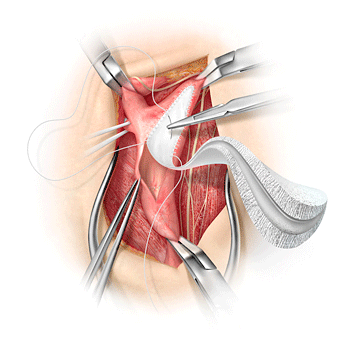
W. L. Gore &Associates (Gore) today announced the launch of the GORE® ACUSEAL VascularGraft for vascular access. Designed for early cannulation within 24 hours afterimplantation, the GORE® ACUSEAL Vascular Graft expands treatment options forearlier removal or possible avoidance of a central venous catheter – a majorsource of infection for hemodialysis patients. The Food and Drug Administration(FDA) cleared the GORE® ACUSEAL Vascular Graft in April 2013.
Earlycannulation is made possible by the unique tri-layer design of the GORE®ACUSEAL Vascular Graft, featuring a low-bleed elastomeric middle membranebetween inner and outer layers of expanded polytetrafluoroethylene (ePTFE).This middle membrane hinders suture line and cannulation needle bleeding andmay reduce the risk of seroma formation and pseudoaneurysm. The GORE® ACUSEALVascular Graft design is kink resistant and flexible at curves while allowingfor precise suturing and anastomotic tailoring.
Therecently completed prospective, non-randomized, multi-center US clinical trialconsisting of 138 subjects demonstrated that the six-month cumulative patencyof the GORE® ACUSEAL Vascular Graft is comparable to that of otherarteriovenous grafts with 84 percent patency compared to 75 percent patency inthe historical control. Results also showed that within 28 days of graftimplantation, 75.6 percent of the implanted GORE® ACUSEAL Vascular Grafts hadbeen successfully cannulated three consecutive times allowing for the potentialfor the central venous catheter to be removed.
"Withearly cannulation, physicians can reduce the number of days patients usetunnelled catheters for dialysis, which in turn will reduce the morbidity andmortality for these patients," said Dr. Marc Glickman, M.D., principalinvestigator and chief of Vascular Services for Sentara Healthcare in Norfolk,Va. "The results of our trial demonstrate that the GORE® ACUSEAL VascularGraft does allow for early cannulation within 72 hours of implantation withoutthe risk of cannulation-related complications such as infection and withoutreducing the patency of the graft."
Dr.Glickman presented Early Cannulation Graft: Results of ACUSEAL Clinical Trialat the 2013 Veithsymposium on Tuesday, November 19, 2013.
The ePTFEluminal surface of the GORE® ACUSEAL Vascular Graft incorporates the CARMEDA®BioActive Surface (CBAS® Surface). This proprietary end-point covalently bondedheparin technology is anchored to the graft surface and imparts proventhromboresistant properties to the vascular graft, while achieving sustainedanti-thrombotic bioactivity on the graft surface for an extended period oftime. CBAS® Surface is the same proven technology introduced with the GORE®PROPATEN® Vascular Graft.
"Thenew GORE® ACUSEAL Vascular Graft represents Gore’s long standing commitment todeveloping innovative graft products that improve patient care," saidCress Whitfield, Sales Leader, Surgical Vascular, Gore Medical. "Byoffering a graft that allows for early cannulation, physicians can providepatients with better treatment options while still benefitting from theuncompromised handling offered by Gore’s vascular grafts."
- Fuente: endovascular.es





