Noticias

- Título: DSM partners with BiO2 Medical to provide ComfortCoat® Coating for the Angel® Catheter
- Fecha: 12-11-2013
Exton, PA (US), 12 November 2013
Partnership offers BiO2 Medical a hydrophilic and lubricious coating to enhance ease of placement and reduce access site trauma for the Angel® Catheter, a central venous access catheter (CVC) with a permanently attached inferior vena cava (IVC) filter.
DSM, a global leader in biomedical materials science and regenerative medicine, today announced that the company has partnered with BiO2 Medical, Inc. DSM will supply its proprietary ComfortCoat® lubricious coating and its coating application expertise for use in BiO2 Medical’s Angel® Catheter.
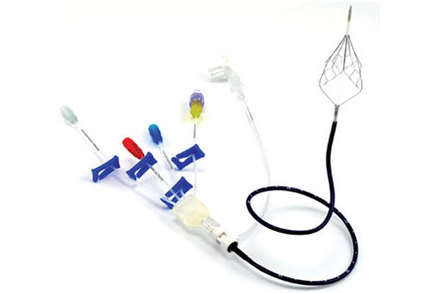
Placed at a patient’s bedside, the Angel® Catheter is intended to provide IVC filter protection from pulmonary embolism (PE) immediately following admission to the Intensive Care Unit (ICU), while simultaneously allowing for central venous access in critically ill patients. PE is a blockage of the main pulmonary artery or one of its branches by a blood clot originating from a different location in the body. A report from Millennium Research Group found that as of 2011, there were more than 250,000 cases of confirmed PE treated in US hospitals; that number increases to 344,000 when patients with symptoms consistent with PE are included.
The unique design of the Angel® Catheter incorporates the PE protection of a retrievable, Nitinol IVC filter, permanently attached to a triple lumen, central venous access catheter. The Angel® Catheter is the first IVC filter to receive CE Mark approval for a prophylactic use indication, in addition to traditional IVC filter and central venous catheter indications.
“This partnership allows our flagship product, the Angel® Catheter, to be easily placed at the patient’s bedside by the attending physician due to DSM’s hydrophilic and lubricous coating technology,” said Jeff Steinmetz, Vice President of Research & Development, BiO2 Medical. “We partnered with DSM due to their highly regarded technical support, responsiveness, and ability to meet project timelines.” “The Angel® Catheter’s hydrophilic coating makes it easy to place and may reduce vessel site trauma, which is important for my critically ill patients,” said Dr. Carl Waldmann, Consultant Anesthetist & Intensive Care, Royal Berkshire NHS Trust, Reading, UK.
“DSM’s proprietary coating technologies and coating processes have a wide range of applications, making them suitable for devices across many medical competences,” said Dr. Hinke Malda, Director of Coatings, DSM Biomedical. “We are proud to see our ComfortCoat® coating contributing to the performance of BiO2 Medical’s Angel® Catheter. The usage in this new catheter not only demonstrates the broad capabilities of our technologies but also underscores how committed DSM and its partners are to addressing unmet market needs with medical innovations.”
The Angel® Catheter has CE Mark approval and is currently commercialized in Europe and the Middle East. It is also the subject of an FDA Early Feasibility Pilot Study Program. Up to 10 patients will be enrolled in the study with initial results anticipated to become available in Q2 of 2014.
- Fuente: endovascular.es





