Noticias

- Título: First patient enrolled in the EVAS Forward global registry
- Fecha: 31-10-2013
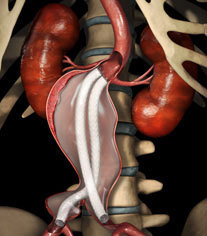
- On 31 October, Endologix announced that the first patient was enrolled in the global registry for the Nellix Endovascular Aneurysm Sealing system. The global registry, is one of a number of clinical studies that make up the broader EVAS Forward clinical programme aimed at establishing clinical and economic evidence for endovascular aneurysm sealing (EVAS).
Andrew Holden, an early Nellix investigator, director of Interventional Services at Auckland City Hospital, associate professor of Radiology, Auckland University School of Medicine in New Zealand implanted the first patient.
“We are pleased to have the first patient enrolled in this important registry. The unique ability of Nellix to fill and seal an aortic aneurysm sac positions it as a significantly improved solution for abdominal aortic aneurysm repair,” said Holden.
“We believe it has the potential to simplify the procedure, improve outcomes for patients, and become a new gold standard for the treatment of abdominal aortic aneurysm. We look forward to collaborating with Endologix and other investigators as we work together to evaluate the full potential of this new technology.”
The Nellix system is designed for the treatment of infrarenal abdominal aortic aneurysms. It is approved for commercial use in Europe and investigational use in the USA is expected shortly. A press release from Endologix states that the Nellix system is targeted to address the direct causes of re-interventions and is intended to expand the treatable patient population.
The EVAS Forward global registry will enroll 300 patients at up to 30 sites with five-year follow-up in Europe and around the world where Nellix is commercially available. - Fuente: endovascular.es





